Why your next trip to the gyno might be different: There’s an alternative to Pap smears
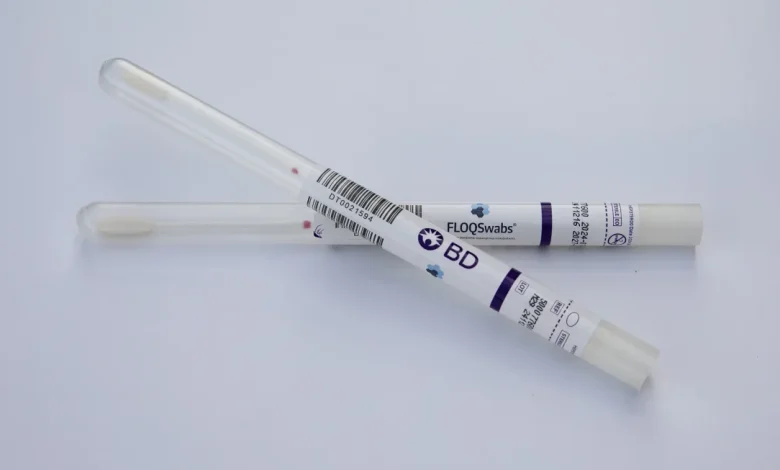
CNN — Currently, the first shipments of self-collection HPV tests for cervical cancer screening are en route to US physician clinics.
The US Food and Drug Administration approved in May the option for patients to obtain their own vaginal samples for cervical cancer screenings, rather than having Pap smears, which use a speculum to screen for the disease, or conventional HPV tests. Patients can now take their own vaginal samples in a medical setting, like an urgent care center, drugstore, or doctor’s office, just as they can take their own urine samples.
There are now two healthcare companies that offer HPV tests that can be utilized with self-collected samples: the biotechnology company Roche and the medical technology company BD. Since the majority of cervical cancers are brought on by the human papillomavirus, or HPV, most cervical cancer checkups include testing for the virus.
According to the business, BD self-collection HPV test shipments started on Thursday. Furthermore, Roche predicts that shipments of its HPV self-collection screening solution will begin this autumn.
The idea behind offering a self-collection approach, according to board-certified gynecologist and vice president of Global Medical Affairs for Diagnostic Solutions at BD, is to increase patient access to screening and consequently, the number of women tested.
The US Centers for illness Control and Prevention estimate that more than 11,000 new cases of cervical cancer are diagnosed in the country each year, and that the illness claims the lives of around 4,000 women. An estimated 50% of occurrences of invasive cervical cancer are found in individuals who have never been screened, and 10% occur in those who have not had a screening within the previous five years.
We know that some of the women who were previously underscreened have benefited from the addition of the self-collection option in other European, Australian, and New Zealand countries. Therefore, the primary objective is to create an approach that would be appealing to women who would not otherwise be tested, according to Andrews.
“Nearly 30% of women are either underscreened or unscreened in the United States specifically,” he stated. “Those are the women who have the highest risk of developing cervical cancer. Women who are not screened within the interval—that is, the suggested time frame for which women are advised to screen—occur approximately two thirds of cervical cancer cases.
Methods to screen for cervical cancer
For women aged 21 to 29, the US Preventive Services Task Force advises screening for cervical cancer every three years using cervical cytology, commonly referred to as a Pap test or Pap smear. The USPSTF recommends screening for cervical cancer every three years for women aged 30 to 65, every five years for women ages 35 to 65, or every five years for women ages 35 to 65 with high-risk HPV testing in addition to cytology.
Gynecologists typically take samples for cervical cytology, HPV testing, or both. Any screening procedure that involves taking samples with a speculum may cause discomfort for certain patients.
In order to identify precancerous or cancerous cells, cervical cytology entails analyzing cervical cells for alterations. The HPV test looks for high-risk HPV infections in cells that can lead to cervical cancer. An HPV and Pap co-test combines the two tests to screen for changes in cervical cells and high-risk HPV.
Approximately 80% of individuals are predicted to contract HPV at some point in their lives. The more than 150 viruses that make up HPV are mainly transmitted through sexual contact. Low-risk strains of the virus typically cause warts, whereas high-risk strains have been linked to an elevated risk of several malignancies, including oropharyngeal, cervical, anal, and penile cancers. Most HPV infections go away on their own in two years, but if the infection persists, health issues like cancer could arise.
When the FDA approved HPV self-collection testing for cervical cancer screening in May, Dr. Karen E. Knudsen, CEO of the American Cancer Society, stated in a news release that “almost all cervical cancers are caused by persistent infection with certain types of HPV.” “By lowering barriers and increasing access to screening, self-collection can help more people identify, treat, and ultimately survive cancer.”
It can hurt or feel awkward for some individuals to have a vaginal sample taken for HPV testing or cervical cancer screening. In order to scrape cells from the cervix, the patient’s doctor vaginally inserts a cold, metal speculum while the patient spreads their legs and keeps their feet in stirrups.
Because a typical Pap smear screening can be uncomfortable, many people may forego scheduled Pap tests to check for cervical cancer.
It has been found that women are much less likely to set the aim of scheduling their first-ever Pap test if they believe the procedure will be uncomfortable. However, reducing discomfort during a Pap test will probably improve screening adherence and acceptance for cervical cancer.
“We can lower the incidence of this malignancy if we can increase screening. It is crucial to have alternative screening modalities or options to increase accessibility and availability, particularly for our marginalized and underprivileged communities. Dr. John Vullo, assistant head of Catholic Health Women’s Health Services and chairman of the obstetrics and gynecology department at Catholic Health’s Good Samaritan University Hospital on Long Island, stated via email that self-screening gives women this additional option.
“While I think it’s crucial to give our patients this new option, I’m relieved that the current guidelines suggest using it under a doctor’s supervision so that the patient can fully comprehend the advantages and restrictions of this new testing,” Vullo stated in part. “By doing this, we can provide them with an additional method of screening for cervical cancer and still give them the opportunity to consult with a provider about other health concerns that are covered in the yearly well-woman exam.”
In order to ensure that the patient doing the test will receive an explanation of the results, a clinician would need to arrange the self-collection screening test for the patient. This would put the patient in contact with their physician, according to Andrews.
A lab would receive the order. After that, the lab would assemble a self-collection kit comprising the screening test itself, a six-inch swab, and instructions. The patient’s doctor’s office would receive the kit by mail. The doctor’s office bathroom might be used by the patient to self-collect a vaginal sample by inserting the swab three inches into the vagina. The sample would then be sent to the lab for analysis by the doctor’s office. More testing is probably advised by the physician if a sample returns positive results.
Home tests are possible in the future
Companies are expecting that self-collection kits will someday be approved for use at home, even though the self-collection approach is currently accessible in healthcare facilities.
According to Andrews, BD has been collaborating extensively with the FDA to produce evidence that supports the use of its self-collection tests at home. The FDA gave “breakthrough device” status to Teal Health, a different business, in May, enabling the FDA to expedite the device’s assessment. The Teal Wand is an at-home cervical cancer screening tool.
Trena Depel, vice president of clinical and regulatory at Teal Health, stated in a news release at the time that “the FDA’s recognition of the Teal Wand as a Breakthrough device acknowledges the important public health benefit that self-collection for cervical cancer screening can have on those who are rarely screened or who do not participate in clinician-based screening for cervical cancer.”
Cervical cancer screening is crucial regardless of the location of the disease because early cases may show no symptoms at all. Unusual discharge or irregular vaginal bleeding may be symptoms of advanced instances. Treatment options for cervical cancer include radiation therapy, chemotherapy, and surgery.
The CDC states that getting an HPV vaccination, quitting smoking, using condoms during intercourse, getting regular screenings, and seeing a doctor if the findings of a screening test are abnormal are some of the most crucial actions women can take to help avoid cervical cancer.