FDA Approves JAK Inhibitor Deuruxolitinib for Alopecia Areata
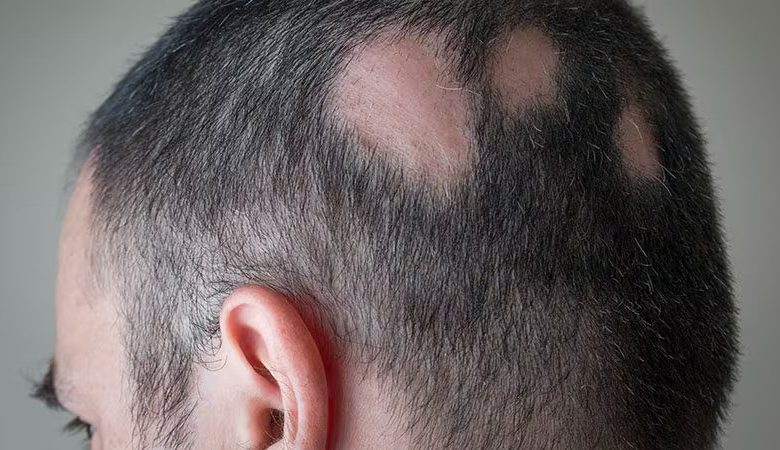
The oral Janus kinase (JAK) inhibitor deuruxolitinib has been approved by the US Food and Drug Administration (FDA) for the treatment of people with severe alopecia areata.
The development is based on data from two pivotal randomized, double-blind, placebo-controlled phase 3 clinical trials: THRIVE-AA1 and THRIVE-AA2, which included 1220 adults with severe alopecia areata enrolled at sites in the United States, Canada, and Europe. The news release from Sun Pharma, the drug’s manufacturer, announced the development on July 25, 2024. According to the Severity of Alopecia Tool (SALT), study participants reported at least 50% scalp hair loss for longer than six months. Additionally, information was gathered from two open-label, long-term extension trials, where patients could re-enroll after the initial 24-week study period ended.
Deuruxolitinib is an oral selective JAK1 and JAK2 inhibitor that is taken twice day in the form of 8-mg tablets. The typical patient participated in the research trials had only 13% of their scalp hair covered at baseline, according to a news release from the business. At week 24, 80% or more of the scalp was covered in hair in over 30% of the deuruxolitinib-using patients (SALT score ≤ 20). Furthermore, at 24 weeks, up to 25% of patients had nearly all of their scalp hair grown back (≥ 90% coverage).
Regarding safety, the information revealed that 3.1% of patients in the phase 2 dose-ranging study and phase 3 randomized placebo-controlled trials stopped using deuruxolitinib 8 mg twice day due to adverse events. In placebo-controlled trials, headache (12.4% vs 9.4% with placebo), acne (10% vs 4.3% with placebo), and nasopharyngitis (8.1% vs 6.7% with placebo) were the three most frequent side effects. Over a hundred individuals took deuruxolitinib for longer than three years.
The third JAK inhibitor and treatment for severe alopecia areata that the FDA has approved is deuruxolitinib. For adults with alopecia areata, baricitinib (Olumiant) was approved in June 2022. Ritlecitinib (Litfulo) was approved in June 2023 for patients 12 years of age and above.
“It is with tremendous excitement that we welcome the FDA’s approval of a third treatment for severe alopecia areata in as many years,” said Nicole Friedland, president and CEO of the National Alopecia Areata Foundation (NAAF), in a statement.